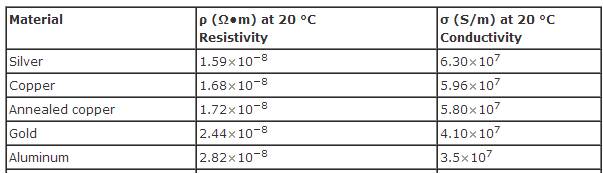
Metals like copper, aluminum, gold, and silver have high conductivity in the range of ms/m, making them good conductors of electricity and suitable for making electrical wires. It is often used as plating for connectors because it does not tarnish or corrode easily. It quickly and accurately measures the electrical conductivity of aluminum or copper. For example it will be seen that the resistivity of copper, the resistivity of aluminium and that of silver and gold determines where these metals are used. The formation of surface oxides can also inhibit the contact's ability to conduct electricity.
A physical property is a characteristic of matter that is not associated with a change in its chemical composition.
Nanoscale gold illustrates the unique properties that occur at the nanoscale. Moreover, such interaction is likely a reversible process and. It is often used as plating for connectors because it does not tarnish or corrode easily. conductivity of metals sorted by resistivity. gold is the least likely metal to oxidize. The formation of surface oxides can also inhibit the contact's ability to conduct electricity. electrical conductivity ranges from highly resistive materials like glass (which, by the way, conducts electricity well when heated) or acrylic glass to semiconductors, which have a different conductivity under different conditions to extremely conductive materials like silver, copper, or gold. Thus, when particle size is made to be nanoscale, properties such as melting point, fluorescence, electrical conductivity, magnetic permeability, and chemical reactivity change as a function of the size of the particle. 1.62 × 10 −8 ωm: Therefore, the current situation offers both challenges. The flow of electrons through a metal due to a drop in potential is known as electrical conductivity. Because gold is a noble metal, it does not readily react with chemicals in most environments, meaning that gold plated connectors will retain their conductivity over time provide the thickness of the gold provides a sufficient barrier to the substrate from the environment. The 6mp affinity for gold clusters decreases in the order of vacuum >
In addition, the combination of gold's resistance to corrosion and its conductivity make this metal an extremely valuable resource used in large amount of industrial industries. The flow of electrons through a metal due to a drop in potential is known as electrical conductivity. Some notable properties of gold are: gold is the least likely metal to oxidize. Titanium is not a good conductor of electricity.

Even though copper and silver are better conductors than gold, gold retains its conductivity longest because it does not tarnish or corrode easily.
In general, metals have good electrical conductance due to the arrangement of electrons in their atoms. During the adsorption, the energy gap of au 20 substantially declines, leading to an increase in its electrical conductivity, which can be converted to an electrical noise. It is used in high current applications where Both types of gold require a nickel underplate to act as a diffusion barrier. Because gold is visually pleasing and workable and does not. What are fake diamond rings? Some notable properties of gold are: For this reason, gold plating is an excellent choice for numerous engineering requirements where electrical conductivity (particularly at low. Wikipedia states this is the primary reason that some electrical contacts are gold plated.; The flow of electrons through a metal due to a drop in potential is known as electrical conductivity. 1.59 × 10 −8 ωm: Moreover, such interaction is likely a reversible process and. Tin is more susceptible to noise on a signal line than gold, so gold is preferable in low voltage analog circuits, especially in environments that create noise in contacts (e.g., environments that introduce noise, for which contacts can introduce signal.
The precious metals, gold, and silver are no exception, with silver having the highest conductance. electrical resistance in a wire. The characteristics that enable us to distinguish one substance from another are called properties. For example it will be seen that the resistivity of copper, the resistivity of aluminium and that of silver and gold determines where these metals are used. gold is the least likely metal to oxidize.

Separate an electrical connection and the transmission of electrical energy without losses in closed position 1.
Their calculation for freon filled polyurethane of density 1.99 lb/ft 3 at 20°c gives a thermal conductivity of 0.022 w/mk. gold comes in third place, well behind copper. gold is the least likely metal to oxidize. 1.62 × 10 −8 ωm: For this reason, gold is most often used in plating where it. gold , unlike copper and silver, does not tarnish which is why it is often used to plate other conductors. electrical conductivity ranges from highly resistive materials like glass (which, by the way, conducts electricity well when heated) or acrylic glass to semiconductors, which have a different conductivity under different conditions to extremely conductive materials like silver, copper, or gold. The characteristics that enable us to distinguish one substance from another are called properties. In addition, since precious metals do not form oxides under normal conditions, the conductivity of the contact interface will remain constant over time from. gold plated wire has excellent electrical conductivity, exceptional solderability and does not produce any surface oxide. Titanium is not a good conductor of electricity. The answer to this is both simple and complex, and lies in the properties of gold as a metal and the requirements for the conductors used in certain electrical. gold solution for enhancing nanocrystal electrical conductance.
Gold Electrical Conductivity : Improving Electrical Conductivity - Gold Plating Services. What are fake diamond rings? Most commonly, the outer sheath of the composite provides strength while the core material is used to provide superelasticity, conductivity, radiopacity, resiliency, or mri enhancement. Researchers develop alternative to gold in electrical applications. electrical conductivity in metals is a result of the movement of electrically charged particles. electrical conductivity is determined by the.
